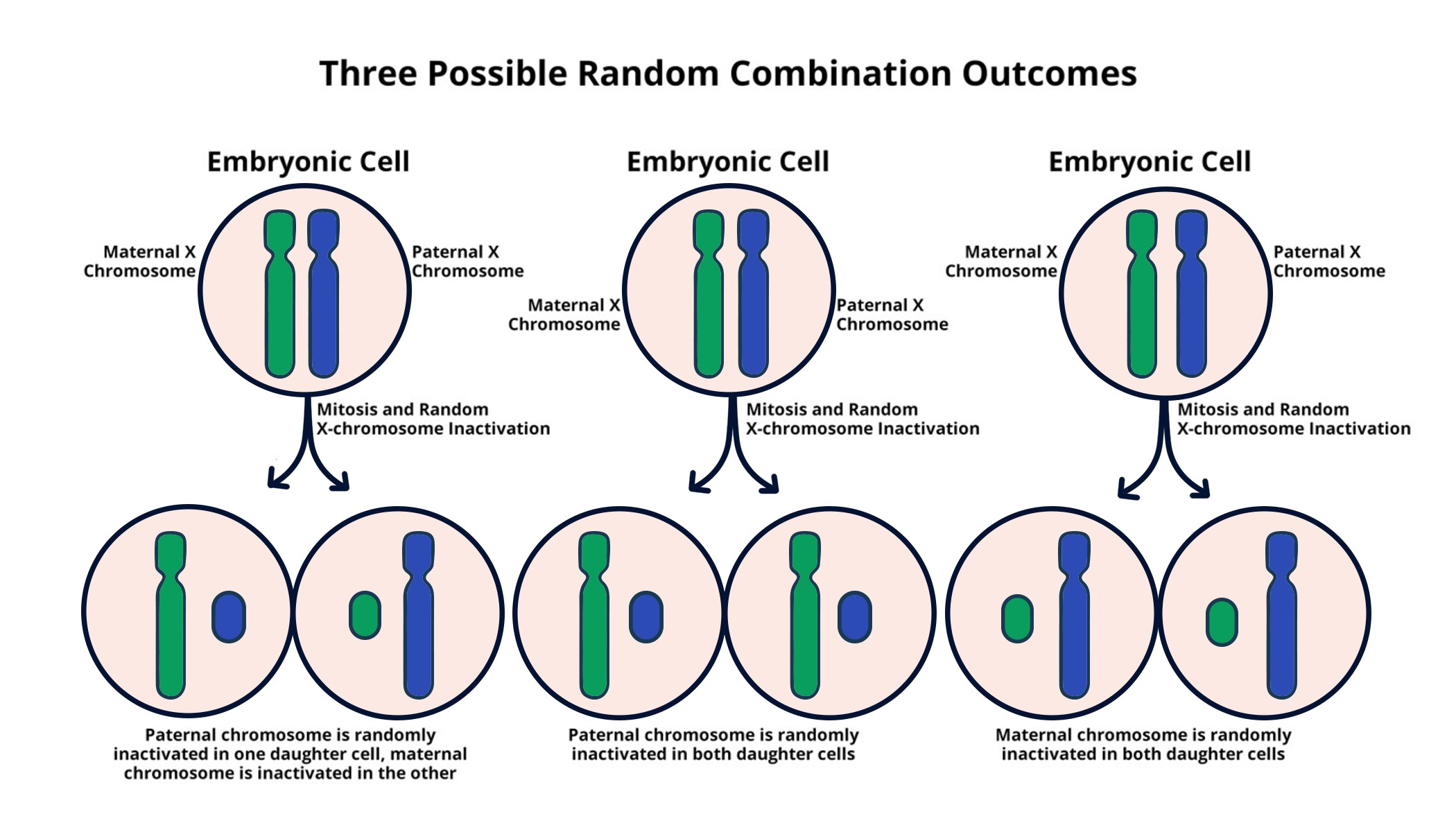
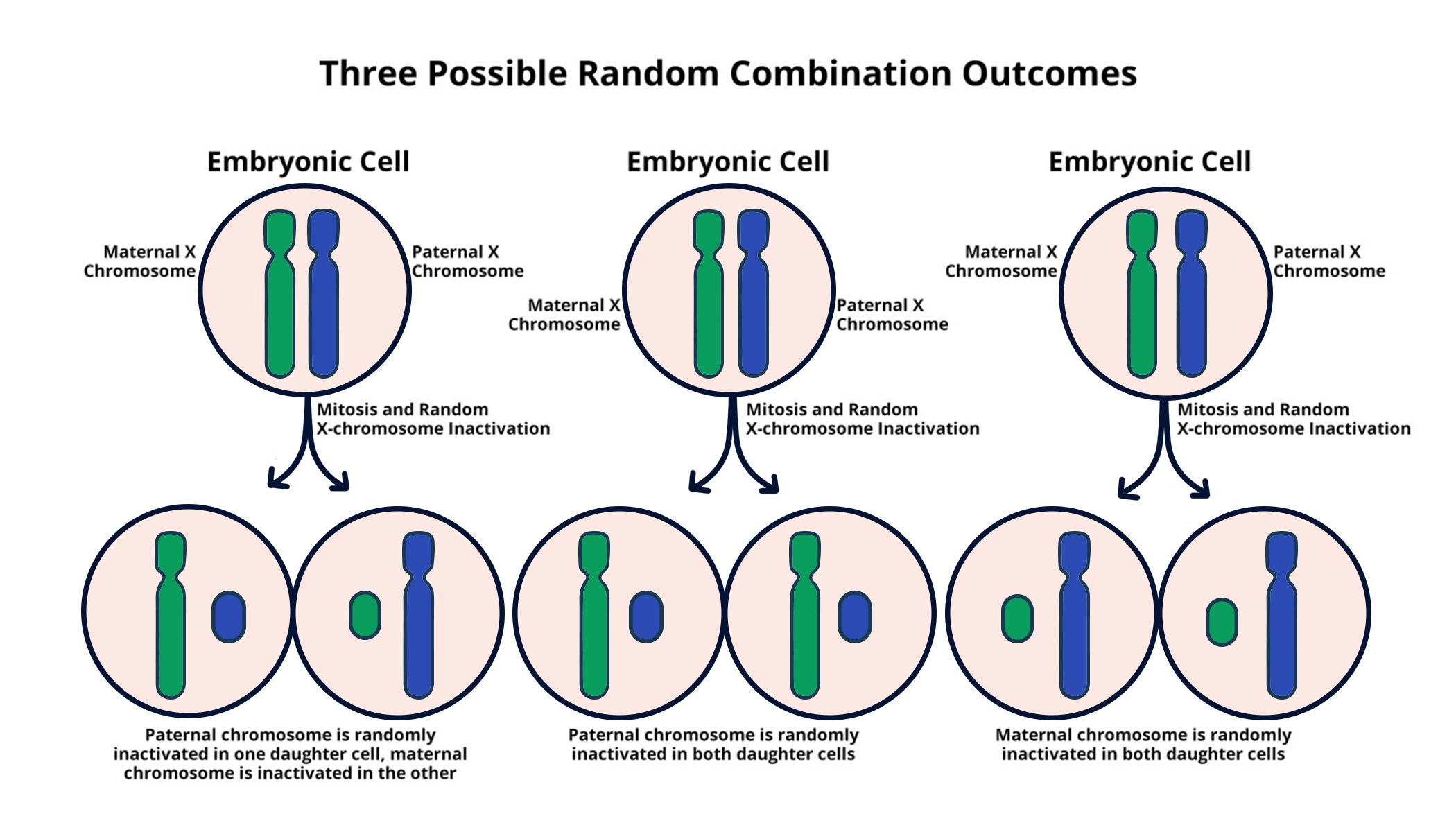
X chromosome inactivation is a fascinating biological process that ensures females maintain balanced expression of genes found on their two X chromosomes. This unique mechanism allows one of the X chromosomes to become largely silent, effectively preventing the overproduction of gene products which could disrupt cellular function. As researchers like Jeannie T. Lee have discovered, this chromosomal breakthrough involves a gelatinous substance that plays a crucial role in the silencing process. Understanding how X inactivation occurs not only illuminates fundamental concepts in genetics but also opens up new pathways for potential treatments for X-linked genetic disorders such as Fragile X syndrome and Rett syndrome. Lee’s research continues to impact the field, as it provides hope for developing therapies that could alleviate the symptoms associated with these conditions and others derived from X chromosome mutations.
The process of X chromosome inactivation represents an essential aspect of genetic regulation in female cells, where it acts to equalize gene expression between the sexes. Known also as Lyonization, this method ensures that cells function effectively despite having two copies of an otherwise redundant genetic blueprint. Jeannie T. Lee’s ongoing research has deeply explored the intricate mechanisms involved, highlighting how a jelly-like substance is critical to silencing one of the X chromosomes. This understanding has far-reaching implications, especially in addressing X-linked disorders such as fragile X syndrome and Rett syndrome. Thus, unraveling the complexities of this process not only contributes to our comprehension of genetic inheritance but also serves as a foundation for future therapeutic innovations.
Understanding X Chromosome Inactivation
X chromosome inactivation (XCI) is a crucial biological process that ensures dosage compensation between males and females by silencing one of the two copies of the X chromosome in females. This intricately regulated mechanism is essential in preventing an overexpression of X-linked genes, which could lead to cellular dysfunction. Understanding the intricacies of XCI helps unravel many of the mysteries surrounding X-linked genetic disorders, such as Fragile X syndrome and Rett syndrome. Noteworthy research, particularly by Jeannie T. Lee at Harvard Medical School, has thrown light on how this inactivation is orchestrated at the cellular level, highlighting the roles of specific RNA molecules and chromosomal interactions.
The process of XCI begins with the production of the Xist RNA molecule, which plays a key role in marking one X chromosome for inactivation. It is surrounded by a unique gelatinous substance, likened to ‘Jell-O,’ that facilitates the silencing of the X chromosome. This interaction is central to the understanding of how X-linked genetic disorders arise when mutations occur on one of the X chromosomes. The mechanism of XCI is not only significant for female biology but also holds therapeutic potential in treating X-linked disorders, promising new avenues for patients suffering from conditions like Fragile X syndrome.
The Implications of Chromosomal Breakthroughs
Recent chromosomal breakthroughs, particularly those discovered by Jeannie T. Lee and her team, herald a new era in the treatment of X-linked genetic disorders. These discoveries elucidate the mechanisms of X chromosome inactivation and reveal new pathways that could be targeted for therapy. The findings hint at the possibility of ‘unsilencing’ inactivated genes, which could restore their function in patients affected by disorders such as Fragile X syndrome, where intellectual disabilities are rooted in genetic mutations. This revolutionary approach could redefine treatment methodologies by unlocking the potential of healthy copies of genes previously rendered inactive.
Moreover, this innovative research sets the stage for the development of future therapies that may not only help females but also males who have mutations in their X chromosome that contribute to conditions like Fragile X syndrome. The strategies being optimized in Lee’s lab could ultimately lead to clinical trials that may introduce new treatment options, paving the way for therapeutic interventions that could alleviate symptoms and improve the quality of life for countless patients. Such advancements mimic a chromosomal breakthrough that could become a cornerstone in the management of X-linked disorders.
Linking X Chromosome Inactivation to Fragile X Syndrome
Fragile X syndrome is intrinsically linked to abnormalities on the X chromosome, making the study of X chromosome inactivation particularly relevant in its context. As the leading genetic cause of intellectual disability, Fragile X syndrome presents unique challenges in gene therapy approaches due to the specific mutations on the affected X chromosome. Research has revealed that XCI can inhibit the expression of healthy genes that are crucial for normal brain development. Jeannie T. Lee’s exploration of this inactivation process offers hope for patients by potentially providing ways to reactivate these critical genes that have been silenced due to XCI.
The insights into X chromosome inactivation mechanisms have set the groundwork for therapeutic strategies aimed at Fragile X syndrome. By targeting the molecular pathways involved in XCI, scientists like Lee are striving to create innovative treatments that address the root causes of this X-linked disorder. This could drastically change the landscape of therapeutic options available for patients, moving from symptomatic relief to potential gene-level corrections that restore healthy gene function in silence. As research progresses, the implications become increasingly positive for individuals and families affected by Fragile X syndrome.
Rett Syndrome: An X-Linked Genetic Disorder
Rett syndrome is another serious neurodevelopmental disorder linked to mutations on the X chromosome, primarily affecting females. This complex condition leads to severe cognitive impairment and loss of motor skills, largely attributable to genetic mutations that occur on the X chromosome. Understanding X chromosome inactivation is vital in the context of Rett syndrome as it provides crucial insights into how gene expression is regulated and how this regulation can be disrupted by mutations like those seen in Rett syndrome. Jeannie T. Lee’s work in XCI may offer paths to re-establishing normal functioning of genes affected by these mutations.
The possibility of targeting X chromosome inactivation pathways to reactivate silent genes holds substantial promise for Rett syndrome therapies. By applying similar methodologies that are being explored for Fragile X syndrome, researchers are hopeful about developing treatments that could un-silence beneficial genes and mitigate the symptoms of Rett syndrome effectively. This groundbreaking approach could pave the way for future research and lead to significant advancements in understanding and treating X-linked disorders, offering hope for improved outcomes for those affected by Rett syndrome.
The Future of Gene Therapy in X-Linked Disorders
The resurgence of interest in gene therapy for X-linked disorders comes at the pivotal intersection of breakthroughs in chromosomal research and therapeutic innovation. The potential for unsilencing genes on the X chromosome provides a framework for developing therapies that could significantly alter the treatment landscape of disorders such as Fragile X syndrome and Rett syndrome. The fundamental understanding gained from the mechanisms of XCI not only opens doors for those suffering from these conditions but may also inspire broader applications within the realm of genetic medicine, focusing on other chromosomal and genetic disorders.
As Jeannie T. Lee and her team continue to refine their approaches and prepare for clinical trials, the focus on ethical implications, safety, and the integration of these therapies into existing medical frameworks cannot be overstated. The future of gene therapy in treating X-linked disorders appears promising, driven by both scientific exploration and the potential for impactful patient outcomes. By leveraging the knowledge gained from decades of research, we stand poised on the brink of potentially transformative therapies that could truly change lives for those afflicted with genetic conditions linked to the X chromosome.
The Role of Jeannie T. Lee in Genetic Research
Jeannie T. Lee has emerged as a pivotal figure in the study of X chromosome inactivation and its implications for treating X-linked genetic disorders. Her research, particularly with the discovery of the significance of the Xist RNA, has opened up novel pathways to understanding not just the biology of XCI, but also its potential therapeutic applications. Lee’s commitment to unveiling the mysteries of XCI has highlighted the impact of chromosomal research in developing treatments for complex disorders like Fragile X syndrome and Rett syndrome. This cross-disciplinary approach illustrates how basic scientific inquiry can lead to significant clinical advancements.
Moreover, Lee’s impactful contributions extend beyond mere theoretical understanding; her work actively seeks to bridge the gap between fundamental research and applicable therapies. By focusing on the mechanisms of XCI and exploring ways to manipulate these pathways, she is at the forefront of a new wave of genetic therapies that hold promise not just for female patients, but for all individuals impacted by X-linked disorders. As the landscape of genetic research continues to evolve, Jeannie T. Lee’s efforts are pivotal in shaping the future of gene therapy and transforming the lives of those affected by these challenging conditions.
Challenges and Mysteries in X Chromosome Inactivation
Despite significant advancements in understanding X chromosome inactivation, many challenges and mysteries remain. The complexity of how inactivation occurs and why certain genes on the X chromosome remain unaffected when others are freed from silencing presents a puzzling conundrum for researchers. Jeannie T. Lee’s investigations into this process point towards a limited capacity of cells to utilize genes, a concept that requires further exploration. Understanding these subtleties is critical as they can have major implications for developing effective therapies for X-linked diseases.
Decades of research have made strides in unveiling the mechanisms governing XCI, yet the nuances of why some genes are preferentially silenced or unsilenced continue to elude scientists. This understanding is essential not just for Fragile X syndrome and Rett syndrome, but for the broader spectrum of X-linked genetic disorders. Fostering collaboration across disciplines and integrating new technologies will be vital in overcoming these challenges and unlocking the complex regulatory networks guiding X chromosome inactivation.
The Importance of Continued Research on X-Linked Genetic Disorders
The ongoing research into X-linked genetic disorders, driven by studies like those conducted by Jeannie T. Lee, is essential for uncovering new therapeutic strategies. Conditions such as Fragile X syndrome and Rett syndrome represent only a fraction of the challenges posed by X-linked mutations. Continued research can reveal vital pathways and mechanisms that can be targeted for potential treatments, thereby enhancing our understanding of how to manipulate gene expression for therapeutic benefit. Such scientific inquiries are foundational for advancing genetic medicine.
Furthermore, as understanding of X-linked disorders expands, the implications for addressing these conditions could lead to broader genetic research applications. The insights gained from studying X chromosome inactivation and its role in gene silencing serve as a precursor for exploring gene therapy options for other chromosomal disorders. It is paramount to keep investing in basic research approaches to unlock, interpret, and manipulate genetic pathways, leading us closer to effective treatments that can fundamentally improve the lives of individuals affected by X-linked genetic disorders.
Frequently Asked Questions
What is X chromosome inactivation and why is it important for females?
X chromosome inactivation (XCI) is a biological process in which one of the two X chromosomes in female cells is randomly inactivated to equalize gene dosage between males and females. This process is crucial as it prevents females from having double the dosage of genes present on the X chromosome compared to males, ensuring proper development and function of cells.
How does X chromosome inactivation relate to Fragile X syndrome?
Fragile X syndrome is an X-linked genetic disorder caused by a mutation in the FMR1 gene on the X chromosome. Since X chromosome inactivation randomly silences one of the two X chromosomes in females, it can result in carriers exhibiting symptoms if the affected X is the one that becomes inactive, leading to developmental issues characteristic of Fragile X syndrome.
What role does Jeannie T. Lee’s research play in understanding Rett syndrome related to X chromosome inactivation?
Jeannie T. Lee’s research has uncovered mechanisms of X chromosome inactivation that shed light on disorders like Rett syndrome, which is caused by mutations in the MECP2 gene, also located on the X chromosome. Her findings on how Xist RNA modifies surrounding chromosomal structures could lead to therapeutic strategies to reactivate the healthy MECP2 gene in affected individuals.
What is the chromosomal breakthrough that Jeannie Lee’s lab discovered regarding X chromosome inactivation?
Jeannie Lee’s lab discovered that a gelatinous substance surrounding chromosomes plays a crucial role in X chromosome inactivation. The interaction between Xist RNA and this substance alters its biophysical properties, promoting the silencing of the X chromosome, which is critical for understanding X-linked disorders and developing therapeutic approaches.
Can therapies developed from X chromosome inactivation research help males with X-linked genetic disorders?
Yes, therapies inspired by X chromosome inactivation research could benefit males with X-linked genetic disorders like Fragile X syndrome. Although males have only one X chromosome, similar mechanisms of gene silencing may apply, allowing for the potential repair or reactivation of mutant genes that affect their health.
How could understanding X chromosome inactivation lead to treatments for X-linked genetic disorders?
Understanding X chromosome inactivation could lead to innovative treatments for X-linked genetic disorders by enabling researchers to unsilence mutated genes. This therapeutic approach, developed by Jeannie Lee’s lab, aims to activate healthy versions of genes that are inactivated, thereby providing relief from conditions such as Fragile X and Rett syndromes.
What future directions does Jeannie T. Lee suggest for her X chromosome inactivation research?
Jeannie T. Lee suggests that her lab will continue to optimize therapeutic approaches targeting X chromosome inactivation, with plans to conduct safety studies and potentially move into clinical trials within the next few years, aiming to develop effective treatments for diseases caused by X-linked mutations.
What are the implications of chromosomal breakthroughs for understanding the mechanics of X-chromosome inactivation?
Chromosomal breakthroughs, such as those discovered by Jeannie T. Lee’s research team, significantly enhance our understanding of X chromosome inactivation mechanics. These insights are crucial for developing targeted therapies for X-linked genetic disorders, as they unravel the complex interactions that facilitate the silencing and potential reactivation of crucial genes.
| Key Points |
|---|
| Females have two X chromosomes, while males have one. X chromosome inactivation is necessary to balance gene dosage. |
| Jeannie T. Lee’s lab has made significant contributions to understanding how X chromosome inactivation occurs. |
| A gelatinous substance, described as ‘Jell-O’, surrounds chromosomes, playing a crucial role in X-inactivation. |
| The RNA molecule Xist alters the properties of the surrounding ‘Jell-O’ to facilitate X chromosome inactivation. |
| This research has implications for treating diseases caused by mutations on the X chromosome, such as Fragile X Syndrome and Rett Syndrome. |
| Efforts are ongoing to develop therapies that could unsilence inactivated genes, providing potential cures. |
Summary
X chromosome inactivation is a vital cellular process that helps balance gene dosage between males and females. This complex mechanism has been extensively studied by researchers like Jeannie T. Lee, who have uncovered key insights into its operation. The findings reveal the involvement of the RNA molecule Xist and a surrounding gelatinous substance, essential for inactivating one of the X chromosomes in female cells. This knowledge not only advances our understanding of genetic regulation but also opens doors to potential treatments for genetic disorders linked to the X chromosome, such as Fragile X Syndrome and Rett Syndrome. Continuing research aims to harness these mechanisms to develop effective therapies, showcasing the promising future of genetic medicine.