The process of X chromosome inactivation represents a fascinating adaptation in female mammals, where one of the two X chromosomes is effectively silenced to balance gene dosage with males, who possess only one X chromosome. This mechanism is crucial for preventing the overexpression of X-linked genes and plays a significant role in understanding genetic diseases, notably Fragile X Syndrome and Rett Syndrome. Researchers, including Jeannie T. Lee of Harvard Medical School, have uncovered the role of the Xist RNA molecule in orchestrating this silencing process, which could pave the way for innovative chromosomal therapies. As scientists delve deeper into the intricacies of X chromosome inactivation, they unveil potential treatments that could unsilence essential genes and offer hope to those affected by debilitating genetic disorders. With the promise of targeted therapies on the horizon, this field of study is not only unraveling the complexities of genetic regulation but also lighting the path toward effective interventions for susceptibility to various diseases linked to the X chromosome.
X chromosome inactivation, also referred to as XCI, is a vital process that ensures dosage compensation between sexes in mammals, particularly in females who have two X chromosomes. This mechanism is instrumental in the development of therapies for various chromosomal disorders, such as Fragile X Syndrome and Rett Syndrome. The Xist RNA molecule is at the heart of this intricate genetic regulation, guiding the silencing of one X chromosome and influencing chromosomal therapies aimed at restoring gene function. Understanding this phenomenon opens up new avenues for addressing genetic diseases, offering hope for innovative treatments that could reverse the effects of mutations found on the X chromosome. Research in this area is expanding our comprehension of how genetic regulation impacts human health, and it emphasizes the importance of exploring XCI-related mechanisms for potential therapeutic advancements.
Understanding X Chromosome Inactivation
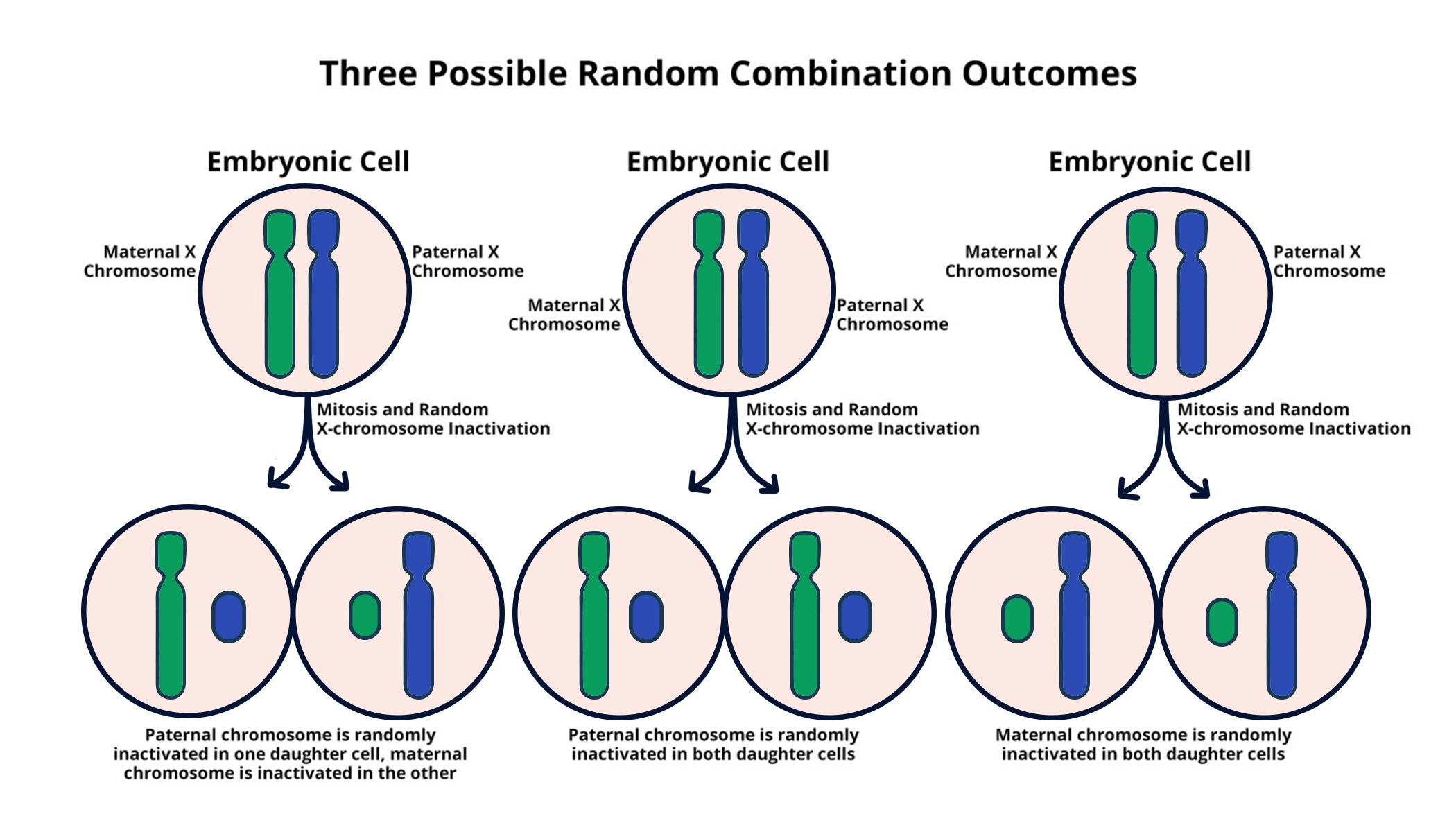
X chromosome inactivation (XCI) is a vital biological mechanism that occurs in female mammals, ensuring that only one of the two copies of the X chromosome is expressed in each cell. This process serves to equalize gene dosage between males, who possess one X chromosome, and females, who possess two. The inactivation is random—meaning that in some cells, the maternal X is inactivated, while in others, the paternal X is silenced. Research led by Jeannie Lee has unraveled some intricacies of how this inactivation unfolds at the molecular level, revealing the crucial role of the Xist RNA molecule. Xist is pivotal in initiating the silencing process as it coats the X chromosome and alters the physical properties of chromatin, which leads to effective gene repression on that chromosome, akin to turning off a light switch.
Understanding X chromosome inactivation is particularly important in the context of genetic disorders. Conditions like Fragile X Syndrome and Rett Syndrome are linked to mutations on the X chromosome, predominantly affecting females while presenting significant challenges in males as well. By learning how to manipulate this inactivation process, researchers hope to develop therapies that can unsilence the affected genes, restoring normal functions. These developments represent a notable shift in the potential treatment landscape for those suffering from genetic diseases, as unlocking healthy genes that are imprisoned in an inactive state could pave the way for novel therapeutic strategies.
The mechanism of XCI is not merely a biological curiosity; it is a crucial component of genetic balance that has implications for the treatment of various genetic disorders. In females, the inactivated X chromosome forms a structure known as a Barr body, which is essential for maintaining genetic stability. As Jeannie Lee notes in her research, the study of XCI has been central to understanding why diseases like Fragile X Syndrome arise and persist. The ability to restore the function of inactivated genes implies a pathway to potentially cure these disorders, a prospect that has spurred extensive research interest and funding, much of which comes from agencies like the National Institutes of Health.
The most exciting aspect of these findings is their translational potential. As research progresses, the discovery of pharmacological interventions that can restore or replace the functional role of mutated genes offers a beacon of hope for millions affected by X-linked diseases. This journey from understanding the basic science of XCI to developing chromosomal therapies highlights a significant step towards resolving some of the most complex genetic disorders affecting human health today.
Potential Therapies for Fragile X Syndrome and Rett Syndrome
Fragile X Syndrome and Rett Syndrome represent significant challenges for affected individuals and their families. Fragile X Syndrome is characterized by intellectual disability, behavioral challenges, and various physical features that often arise from a mutation in the FMR1 gene located on the X chromosome. The link between these disorders and the underlying mechanisms of X chromosome inactivation has led researchers to explore potential therapies that target these specific genetic faults. Current studies suggest that enhancing gene expression from the inactivated X chromosome could alleviate the symptoms associated with these disorders, providing a promising avenue for future treatments.
Jeannie Lee’s lab has been at the forefront of this research, exploring ways to pharmacologically manipulate XCI to reactivate silenced genes. By leveraging the properties of Xist RNA and understanding how it interacts with chromatin, researchers are developing compounds that facilitate the unsilencing of genes crucial for brain function and development. The expectation is that these therapies can be optimized and eventually transitioned to clinical trials, giving hope to families affected by Fragile X Syndrome and Rett Syndrome. The clinical promise offers a significant motivating factor for ongoing research aimed at translating science into tangible medical outcomes.
The complexity of these genetic diseases requires a multi-faceted approach; therefore, combination therapies are being explored as potential treatments for these disorders. Recent advancements in chromosomal therapies aim to utilize engineered viruses or nanoparticles to deliver therapeutic agents that can modify gene expression on the X chromosome. These innovative strategies are designed not only to generate a response in the presence of diseases like Fragile X Syndrome but also to minimize unwanted effects on healthy X-linked genes. This precision in targeting could revolutionize how we approach neurodevelopmental disorders at their genetic roots and open up pathways for other genetic conditions linked to the X chromosome.
As researchers like Jeannie Lee continue to unveil the details of X chromosome inactivation, the translational impact of their work becomes increasingly apparent. The collaboration of basic research with clinical applications is creating a dynamic pipeline, giving rise to strategies that might one day lead to definitive cures for genetic diseases that have long eluded effective treatments. This ongoing research emphasizes the potential of scientific inquiry to illuminate pathways toward better health outcomes for those affected by genetic disorders.
The Role of Xist RNA in Gene Regulation
Xist RNA plays a central role in the process of X chromosome inactivation, making it a key player in understanding gene regulation for X-linked genetic diseases. When Xist RNA is transcribed from the X chromosome, it coats the chromosome, initiating a series of biochemical events that result in gene silencing. This process not only highlights the function of non-coding RNAs in cellular regulation but also underscores the complexity of genetic control mechanisms. Jeannie Lee’s research has demonstrated how Xist alters the physical and chemical environment around the X chromosome, facilitating the recruitment of various regulatory proteins that contribute to the inactivation process.
Understanding the molecular details of how Xist RNA interacts with the chromatin structure offers pathways for developing targeted therapies aimed at reactivating silenced genes associated with diseases like Fragile X Syndrome. By refining our knowledge of this regulatory process, scientists can create therapeutic interventions that might restore function to genes that have been rendered inactive due to mutations. Such advancements represent a crucial step toward rebalancing genetic contributions to health and disease.
Moreover, research into Xist RNA is continuously revealing its additional roles beyond mere inactivation. In the context of chromosomal therapies, manipulating Xist and its pathways opens up potential avenues for future treatments that aim to fine-tune gene expression. Researchers are already investigating ways to harness or modify Xist RNA to develop strategies that could reactivate the healthy allele of affected genes without disrupting the overall balance of gene dosage. This could be particularly beneficial in disorders where only one allele of a gene is mutated, as seen in Fragile X Syndrome and Rett Syndrome.
In summary, studying Xist RNA and its impact on the X chromosome provides invaluable insights into gene regulation mechanisms. These insights not only contribute to our understanding of fundamental biological processes but also hold the key to unlocking innovative therapies for some of the most challenging genetic disorders associated with the X chromosome. As research progresses, the potential to apply this knowledge in clinical settings grows, promising advances in the treatment of genetic diseases.
Implications of Chromosomal Therapies for Genetic Disorders
Chromosomal therapies represent a groundbreaking approach to treating genetic disorders, especially those associated with the X chromosome. These therapies leverage the principles learned from X chromosome inactivation to target and manipulate gene expression at a chromosomal level. The potential implications for conditions like Fragile X Syndrome and Rett Syndrome are profound, as they offer the hope of correcting genetic deficiencies at their source rather than simply managing symptoms. Understanding how inactivated genes can be safely reactivated opens doors to novel treatment methods that could lead to permanent solutions for individuals affected by these conditions.
Researchers are actively exploring how chromosomal therapies can be tailored for specific genetic mutations, enhancing the precision of treatment. By focusing on the specific characteristics of diseases tied to the X chromosome, these therapies can be developed to minimize side effects while maximizing effectiveness. As Jeannie Lee’s lab continues to innovate in this area, the potential to translate these findings into clinical practices becomes an ever-closer reality, providing hope for those who suffer from genetic diseases.
Moreover, the broader implications of chromosomal therapies extend beyond just treating existing conditions. By elucidating the mechanics of X chromosome inactivation and the role of molecular players like Xist RNA, scientists aim to develop a foundation for addressing other genetic disorders linked to chromosomal abnormalities. This understanding lays groundwork for potential preventive strategies, enabling the medical community to consider interventions before the onset of symptoms. As ongoing research progresses, the eventual integration of chromosomal therapies into mainstream medicine could revolutionize the landscape of genetic disease management and prevention, shifting from reactive to proactive healthcare solutions.
Future Directions in Genetic Research
The evolution of genetic research, particularly in the field of X chromosome inactivation, is poised to revolutionize our approach to treating various genetic disorders. As scientists like Jeannie Lee make strides in understanding the underlying mechanisms, the focus will likely shift toward therapeutic development that can cater to the specific needs of individuals suffering from conditions like Fragile X Syndrome and Rett Syndrome. The goal is not only to silence the effects of these diseases but also to work towards curing them by navigating the complexities of gene expression regulation.
Future research directions may involve exploring the relationship between chromosomal configurations and gene function across different species, evaluating how evolutionary tactics influence genetics. This comparative approach could yield novel insights into therapeutic strategies, enhancing our ability to tackle genetic diseases effectively. Additionally, there is a growing interest in personalized medicine, where understanding an individual’s specific genetic makeup will guide tailored therapeutic interventions.
Furthermore, advancements in gene editing technologies, like CRISPR/Cas9, combined with insights gained from chromosomal therapies, could open new doors in the treatment of X-linked genetic diseases. As researchers refine these tools, the ability to correct genetic mutations directly at the source becomes increasingly viable. This paradigm shift in genetic research will not only provide treatments for existing disorders but also enhance our understanding of gene regulation, chromosomal dynamics, and their implications in human health. As the capability to manipulate the genetic landscape becomes more sophisticated, the impact of this research on public health and disease prevention will be profound, potentially reshaping how future generations approach genetic disorders.
Challenges in Unlocking X Chromosome Potential
Despite the promising advancements in understanding X chromosome inactivation and its implications for genetic disorders, several challenges remain. One significant hurdle is the complexity of the X chromosome itself, which harbors numerous genes that may interact in unforeseen ways. The intricate relationship between gene concurrency and environmental factors complicates the straightforward application of chromosomal therapies to treat conditions like Fragile X Syndrome and Rett Syndrome. Jeannie Lee’s team acknowledges these complexities, emphasizing the need for thorough research and testing before moving to clinical applications.
Another challenge lies in ensuring the safety and efficacy of any potential therapies. As researchers work to unsilence inactivated genes, the risk of inadvertently affecting other crucial genes on the X chromosome persists. Understanding the mechanisms behind these interactions is paramount. Ongoing studies aim to map out the functional landscape of the X chromosome intricately, ensuring that therapeutic interventions will not provoke adverse effects or further complications.
Moreover, ethical considerations in genetic therapy must be addressed as the scientific community moves towards clinical applications. The ability to manipulate genes raises significant ethical questions about the long-term impacts on individuals and future generations. As techniques become available to potentially ‘enhance’ genetic attributes, the discourse surrounding genetic equity, access to treatments, and the societal implications of these advancements will become increasingly critical.
Navigating these challenges will require a collaborative approach, combining insights from genetics, bioethics, and clinical practice to forge a path forward. As researchers, policymakers, and healthcare providers engage in this dialogue, the goal remains clear: to leverage our growing understanding of the X chromosome’s potential for the betterment of those living with genetic disorders. The balance between innovation, safety, and ethical responsibility will shape the future landscape of genetic research and therapy.
Frequently Asked Questions
What is X chromosome inactivation and why is it important in genetic diseases?
X chromosome inactivation is a biological process where one of the two X chromosomes in female cells is silenced to prevent an excess of gene expression. This mechanism is crucial for understanding genetic diseases like Fragile X Syndrome and Rett Syndrome, as it can affect the availability of gene expression from the X chromosome, directly impacting the development of therapies for these conditions.
How does X chromosome inactivation relate to Fragile X Syndrome?
Fragile X Syndrome is a genetic disorder caused by mutations on the X chromosome. X chromosome inactivation plays a key role because, in females, one X chromosome is silenced, which can prevent the healthy version of the gene from being expressed if it is located on the inactivated chromosome. Understanding X inactivation can lead to innovative treatments aimed at unsilencing these critical genes.
What role does the Xist RNA molecule play in X chromosome inactivation?
The Xist RNA molecule is vital for X chromosome inactivation. It coats the X chromosome and alters the chromosomal environment, effectively silencing its expression. By adjusting the properties of surrounding chromatin, Xist helps facilitate the inactivation process, which is crucial for controlling gene expression related to genetic diseases.
Can therapies based on X chromosome inactivation help treat Rett Syndrome?
Yes, therapies targeting X chromosome inactivation have the potential to treat Rett Syndrome. By understanding how to unsilence the inactivated X chromosome, researchers aim to restore function to the healthy genes affected by mutations, offering hope for new treatments for this neurodevelopmental disorder.
What advances have been made in chromosomal therapies related to X chromosome inactivation?
Recent advances in chromosomal therapies focus on utilizing mechanisms of X chromosome inactivation to develop treatments for genetic diseases like Fragile X Syndrome and Rett Syndrome. Researchers have made significant strides in unsilencing X-linked genes in lab settings, paving the way for potential clinical trials aimed at delivering effective therapies for those diseases.
How does understanding X chromosome inactivation help in developing treatments for genetic disorders?
Understanding X chromosome inactivation provides insights into the regulation of gene expression on the X chromosome, which is crucial for diseases stemming from X-linked mutations. By exploring how Xist and surrounding chromatin interact, scientists can create strategies to reactivate healthy genes and mitigate the effects of genetic disorders such as Fragile X Syndrome.
| Key Point | Details |
|---|---|
| X Chromosome Inactivation | Females have two X chromosomes, while males have one. To balance gene dosage, one X chromosome in females is inactivated. |
| Role of Xist | Xist is an RNA molecule that initiates the inactivation process by modifying the chromosomal environment. |
| Biophysical Changes | Xist alters the properties of the ‘Jell-O’ surrounding chromosomes, allowing other molecules to access the X chromosome for inactivation. |
| Therapeutic Potential | Uncovering the X chromosome’s inactivation process could lead to treatments for genetic disorders like Fragile X and Rett syndrome. |
| Research Support | The research has been supported for over 25 years by the National Institutes of Health, moving from basic science to potential therapies. |
Summary
X chromosome inactivation is a crucial biological process that ensures proper gene dosage in females, who possess two X chromosomes compared to males with one. This mechanism is vital for understanding and potentially treating genetic disorders associated with the X chromosome. Recent advancements in research by Jeannie T. Lee’s lab reveal insights into how Xist facilitates the silencing of one X chromosome, opening avenues for therapeutic interventions for conditions like Fragile X and Rett syndrome. Such breakthroughs underscore the significance of X chromosome inactivation not only in basic biology but also in developing future genetic treatments.